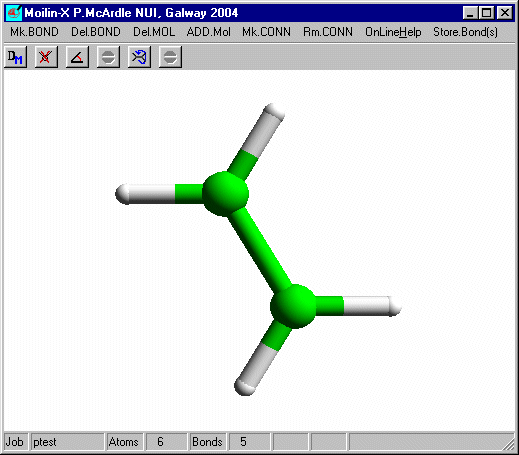
Click one of the H atoms and on the build dialog set the Atom Type to Br and select Add isolated atom.
Click OK and you should get
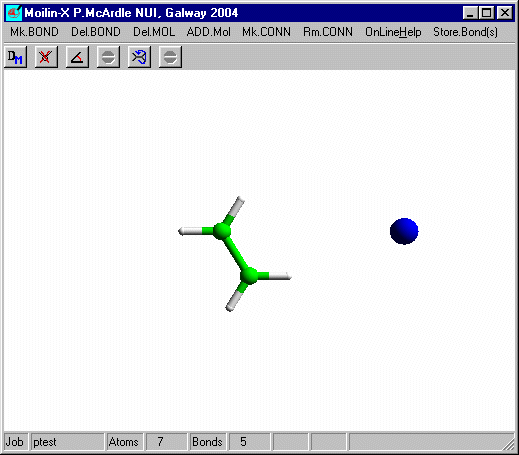
Click the right mouse button to leave build mode and click the
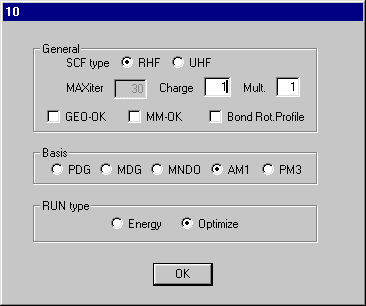
Set Charge to 1 on dialog10 and click OK and Click RunMopac. Rotate the optimized structure and it should look like that shown here.
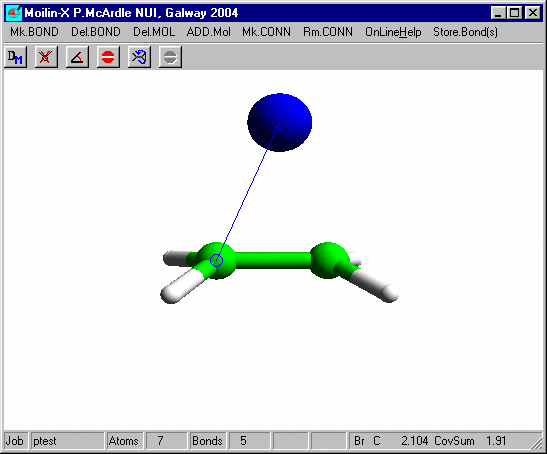
Measure the distance from the Bromine to one of the carbons and you will see that it is slightly longer than the sum of the covalent radii. The bromine is equi-distant from the carbons and the "ethene" is no longer planar. To get the second Br to add correctly you need to tilt the structure slightly before adding the second bromine.

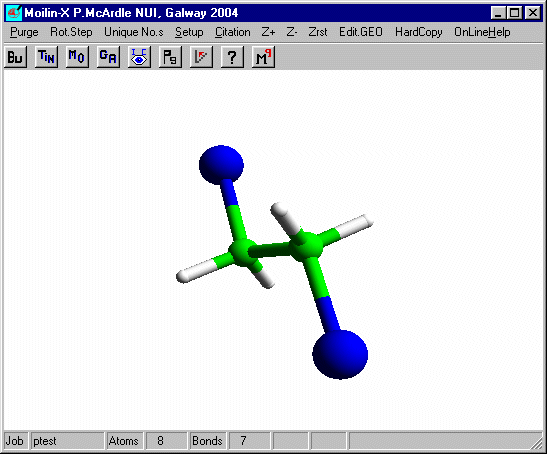
In build mode click one of the hydrogens and add an isolated Br as before and after optimisation using Mopac defaults you should have.

Measure the distance from the bromines to the carbons. It is now a normal covalent bond and the H-C-H angles at C are 109� and typical of tetrahedral carbon. You can put these bonds in using Mk.CONN for cosmetic purposes.
Note on the nature of the calculations. Ethene and bromine react in solution but simple quantum mechanical calculations are performed in the "gas phase" where ions are much less stable. This is why it is necessary to contrive the two steps of the reaction to some extent. However this demonstration gets across the important geometry changes that take place at carbon during the reaction. The Bond Rotation Energy Profile for the C-C bond in 1,2-dibromoethane is similar to that of 1,2-dichloroethane.
Back