A Simple Molecular Orbital Description of the Bonding in
Ferrocene
It is possible to derive a reasonable bonding model by
considering the interaction of the molecular orbitals fromed by the p-p
orbitals of two
cyclopentadienyl (Cp) anions and an Fe2+ cation. The strategy is to
consider the orbital interactions in three steps:
1 the orbitals of one ring,
2 the orbitals of two rings combined
3 the orbitals of two rings interacting with Fe2+.
The Orbitals of One Ring
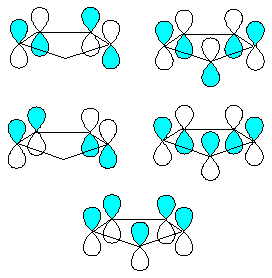
The ring and the hydrogens are bonded by an sp2 framework. This
leaves one pz orbital on each carbon unused. There are five ways to combine the
five pz orbitals. The most symmetrical combination has the lowest energy and one
node (A1' symmetry, D5h point group). Higher in energy lie a degenerate pair which have two nodal
planes (E1' symmetry). At the highest energy are a pair of orbitals with three
nodes. There are 6 p-electrons in Cp- and thus the three lowest
energy orbitals are filled. Build Cp- using Moilin with the ptest
jobname and run Mopac to obtain orbital plots you will see that the symmetrical
molecular orbital is M.O. 9
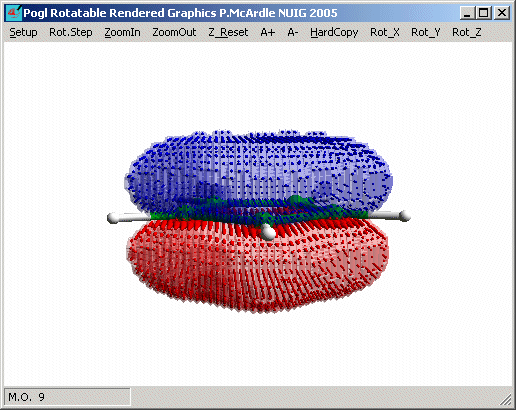
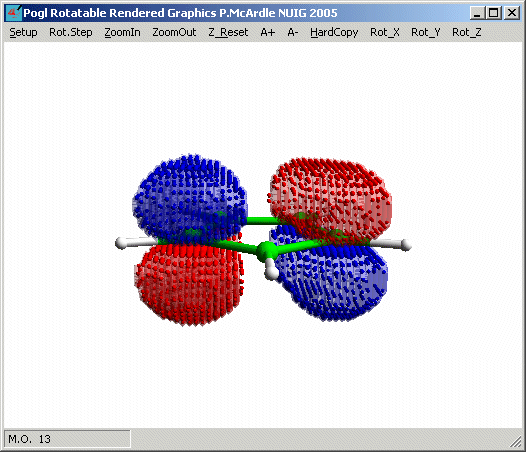
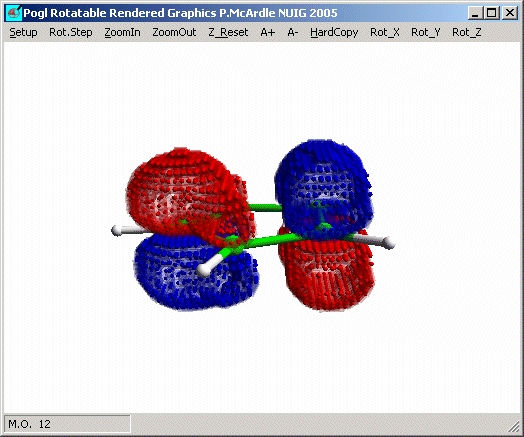
and that M.O. 12 and 13 are the other two filled p-bonding
orbitals. Notice their vertical nodes are orthognal (i.e. at 90� to each other)
The Orbitals of Two Rings
To examine the orbitals of two rings combined. Purge the display and load ferrocene_d5h from
the Moilin library into the ptest job. Delete the Fe atom and use Iconc
to calculate the energy of the system. Do not forget to set the charge to -2.
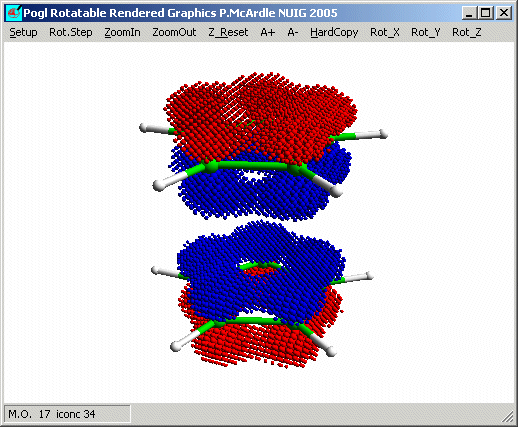
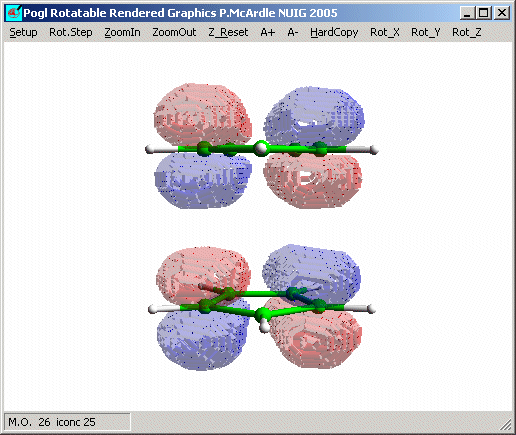
Look at the orbitals shown here: M.O. nos. 17, 18, 25 and 26. Molecular orbital
26 is the HOMO and it has the same energy as (is degenerate with) 25. You can
see this degeneracy if you examine the energies in the LST file. These four
orbitals are clearly combinations of the orbitals of one ring. Orbital 17 is the
most symmetrical way to combine two A1' orbitals and 17 has A1'
symmetry. Notice the hole in the centre of the blue density. The outline option
was deselected for this plot.
M.O. 17 the most symmetrical A1 combination.
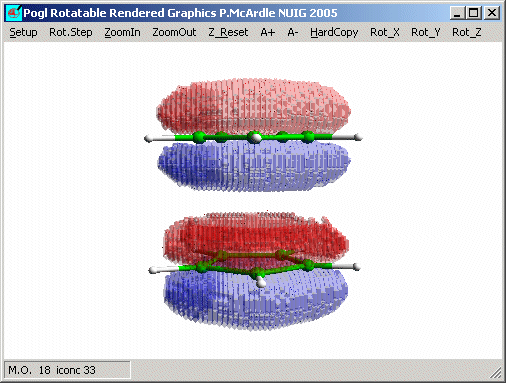
The less symmetrical A1' combination has A2' symmetry.
An E1' combination

Another E1' combination.
Iron Orbital Interactions with the Two Ring Orbitals
The valence orbitals of Iron are the 4s, 4p and 3d
orbitals. The 4s and the 3dz2 have the same
symmetry type as the most symmetrical A1' combination. The 3dxz and 3dyz
have the same symmetry type as the E1' combinations. Thus some of the
possible interactions are
4s or 3dz2 with the most symmetrical A1'
combination.
The 4pz with less symmetrical A2' combination.
The 3dxz and 3dyz with the E1' combinations.
Purge the display and load ferrocene_d5h from
the Moilin library and use Iconc to calculate the energy of the
system. Plot the molecular orbitals and look at the HOMO.
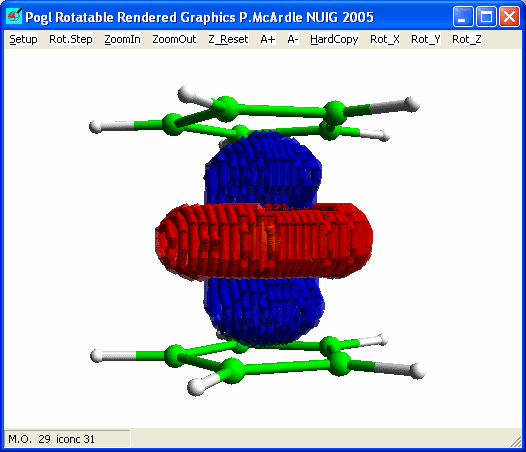
The ferrocene HOMO looks like the 3dz2 and
there is not much overlap with the ring orbitals. The hole in the centre of the
blue density above is the reason for this poor overlap. The 4pz A2' interaction
also has poor overlap for the same reason.
Plot M.O. 23 (de-select outline) and you should see.
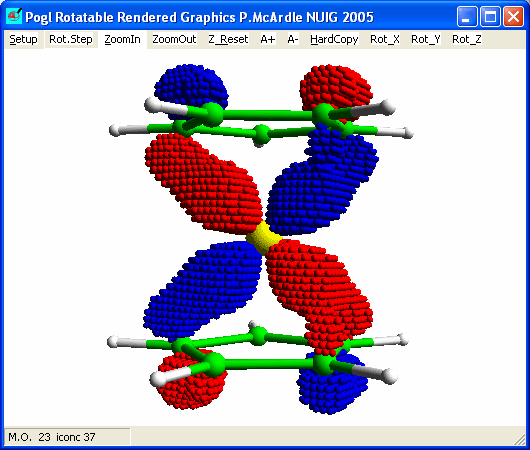
This is dxz - A1' overlap and in combination with its
orthogonal dxz - A1' interaction is the principal bond
holding the rings and the iron together.
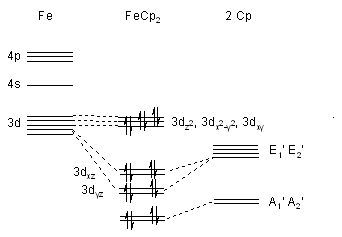
There is general agreement that the E1 - E1 interaction is
the most important one and that the other interactions are non-bonding or
anti-bonding. Thus 18 valence electrons are in bonding or non-bonding levels.
The analysis of Fe - Cp bonding is similar in both the
eclipsed (D5h) and staggered (D5d) structures and there is
not much energy difference between them.
Back










